Results for 'Antineoplastic agents'

Leadership Lab: How To Spot When Employees Are About To Walk Away
Oct 22nd • 7 mins read

Does biomarker use in oncology improve clinical trial failure risk? A large-scale analysis
Feb 23rd • 8 mins read

The First 2 Years of Biosimilar Epoetin for Cancer and Chemotherapy-Induced Anemia in the U.S.: A Review from the Southern Network on Adverse Reactions
Mar 12th • 7 mins read
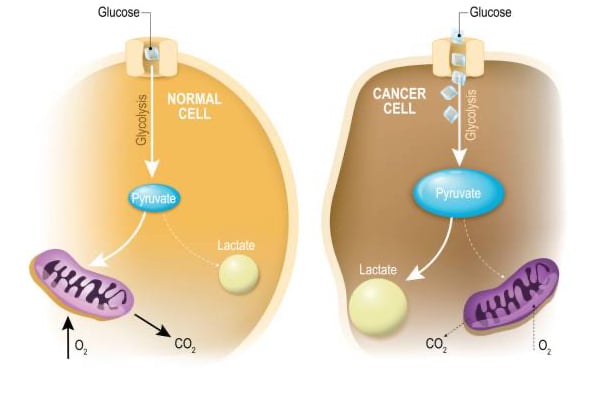
“Oncometabolism: The switchboard of cancer: An editorial”
Feb 1st • 1 min read
Loose Regulatory Standards Portend a New Era of Imprecision Oncology
Dec 1st • 4 mins read
Lessons learnt from scoring adjuvant colon cancer trials and meta-analyses using the ESMO-Magnitude of Clinical Benefit Scale V.1.1
Sep 6th • 17 mins read
Quantitative Clinical Pharmacology of T‐Cell Engaging Bispecifics: Current Perspectives and Opportunities
Nov 18th • 15 mins read
Clinical benefit of immune checkpoint inhibitors approved by US Food and Drug Administration
Aug 31st • 16 mins read

Clinical development success rates and social value of pediatric Phase 1 trials in oncology
Jun 21st • 28 mins read

Assessment of Clinical Trials Supporting US Food and Drug Administration Approval of Novel Therapeutic Agents, 1995-2017
Apr 21st • 20 mins read

Publicly accessible evidence of health-related quality of life benefits associated with cancer drugs approved by the European Medicines Agency between 2009 and 2015
Feb 23rd • 12 mins read
Rise of Antibody-Drug Conjugates: The Present and Future
May 25th • 20 mins read
Pivotal Considerations for Optimal Deployment of Healthy Volunteers in Oncology Drug Development
Oct 31st • 20 mins read
Publication statuses of clinical trials supporting FDA-approved immune checkpoint inhibitors: a meta-epidemiological investigation
Oct 24th • 18 mins read

Prediction of Drug Approval After Phase I Clinical Trials in Oncology: RESOLVED2
Sep 20th • 12 mins read

The rise of oncology biosimilars: from process to promise
Aug 23rd • 18 mins read

Cost per Event Averted in Cancer Trials in the Adjuvant Setting From 2018 to 2022
Jun 10th • 30 mins read

Comparison of Long-term Survival Benefits in Trials of Immune Checkpoint Inhibitor vs Non-Immune Checkpoint Inhibitor Anticancer Agents Using ASCO Value Framework and ESMO Magnitude of Clinical Benefit Scale
Jul 10th • 12 mins read

Uptake of Oncology Biosimilars: Managed Care Strategies to Improve Value-Based Care Systems
Jul 7th • 25 mins read

Oncology biosimilars: New developments and future directions
Nov 25th • 30 mins read
